Off the Pacific Northwest coast in 2005, the baby oysters quietly went missing. At first, shellfish farmers thought that the mass deaths of oyster larvae were a fluke of nature. When the oysters again failed to reproduce in successive years, alarm bells were finally rung. Today, marine scientists have confirmed what many farmers have suspected for a while—the culprit is growing levels of acidic seawater.
To understand what’s happening, we need to take a step back in time. Since the start of the Industrial Revolution, humans have been releasing unprecedented quantities of carbon emissions into the air. While much of these greenhouse gases remain in our atmosphere, about 1/3 of the gases are actually absorbed by the ocean, which acts as a global carbon sink. When in contact with water, carbon dioxide forms carbonic acid, increasing the acidity and fundamentally changing the chemistry of the ocean.
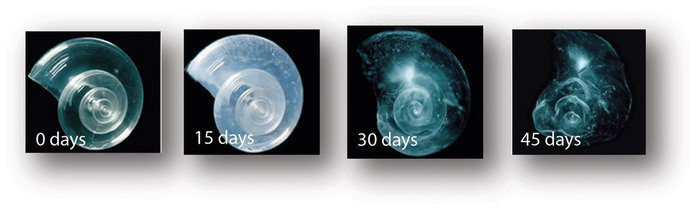
Source: NOAA. Pteropods are tiny, free-swimming sea snails. These photos show the deterioration of a pteoropod shell when place in seawater with the pH levels projected for 2100.
Shellfish are the aquatic canary in the coal mine because they are especially sensitive to changes in the pH of water. Calcium carbonate is used to form hard shells, and when there is an increase in carbon dioxide in water, the number of free carbonate ions is depleted. In addition, aragonite—used to bind calcium and carbonate ions together—dissolves in acidic conditions, meaning the shell will dissolve entirely. Over the last century, the pH of our oceans has decreased from 8.16 to 8.07 today. This decline in pH of 0.1 may not seem like much, but with the logarithmic pH scale, this means there are 30% more hydrogen ions in the water, a significant difference.
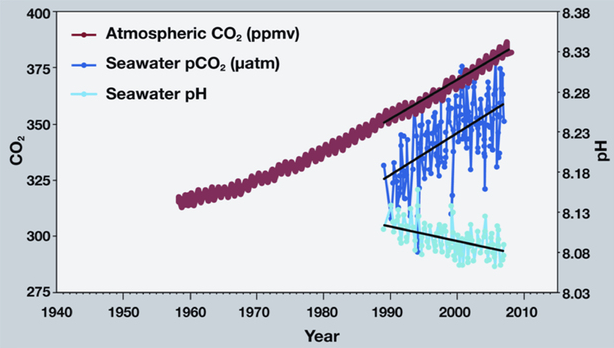
Source: NOAA. This graph shows the correlation between rising levels of carbon dioxide in the atmosphere and in the ocean. As CO2 accumulates in the ocean, the pH decreases.
For years, scientists have been warning of the unintended consequences of releasing greenhouse gases, and now we can see tangible effects with the failure of West Coast oyster larvae to survive. What’s even more worrisome is that the acidification is likely to accelerate in coming years. The deep waters that upwell off the coast of the Pacific Northwest are approximately 50 years old. Carbon dioxide emissions have increased dramatically in the last few decades, meaning that as water from subsequent decades rises to the surface, the oceans are bound to become even more acidic.
For one oyster farmer, the solution was to decamp from the Pacific Northwest for more basic pastures. Rather than depend on oyster seed from WA hatcheries, Goose Point Oysters decided that they were going to build a new hatchery in Hawaii, where the waters have not been impacted by ocean acidification yet. The hatchery is now up and running, but it’s hard to say if the new location will run into acidification issues a decade or so down the road.
It may be too late to stop the 50-year-old waters from upwelling, but we can certainly take measures to cut our carbon emissions now. We must also take urgent steps to protect our shellfish industries through research on pH buffering and vigilant monitoring of incoming seawater. Otherwise, oysters on the half shell may soon be a fond memory.
